MAY 2020, SPECIAL FEATURE
An increasing number of options are available for pharmaceutical companies seeking integrated scientific advice between regulators and health technology assessment (HTA) bodies, along with patient involvement, to optimize their evidence development program and ensure timely patient access to new, transformational medicines. As the array of opportunities for advice continues to evolve in terms of both scope and process, sponsors considering advice need continual, timely insight into how to select the best program(s) for their assets and which programs can be used based on program availability.
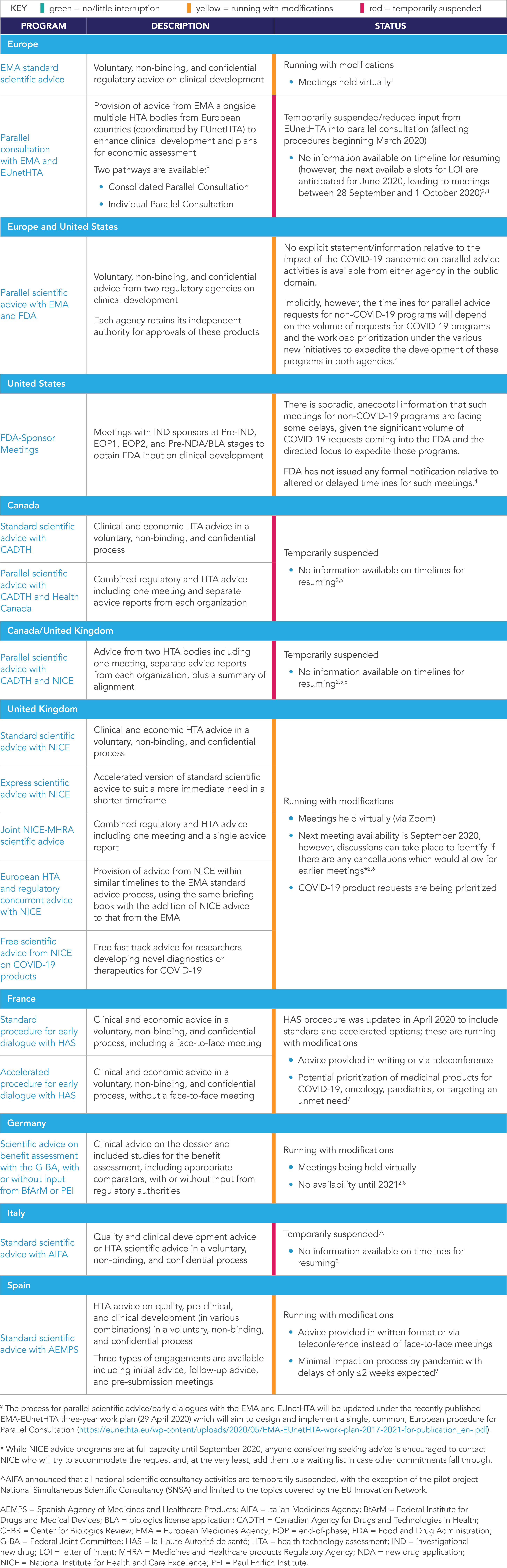
The COVID-19 pandemic has also resulted in changes to the status of advice programs as regulators and HTA bodies prioritize resources to respond to outbreak-related requests and healthcare practitioners provide care to those affected by the pandemic. While there are resulting delays and disruptions to various advice programs across stakeholders and markets, there are still options available for sponsors seeking advice. A summary of the status of key advice programs in North America and Europe is presented in Table 1.
Scientific Advice Options during the COVID-19 Pandemic
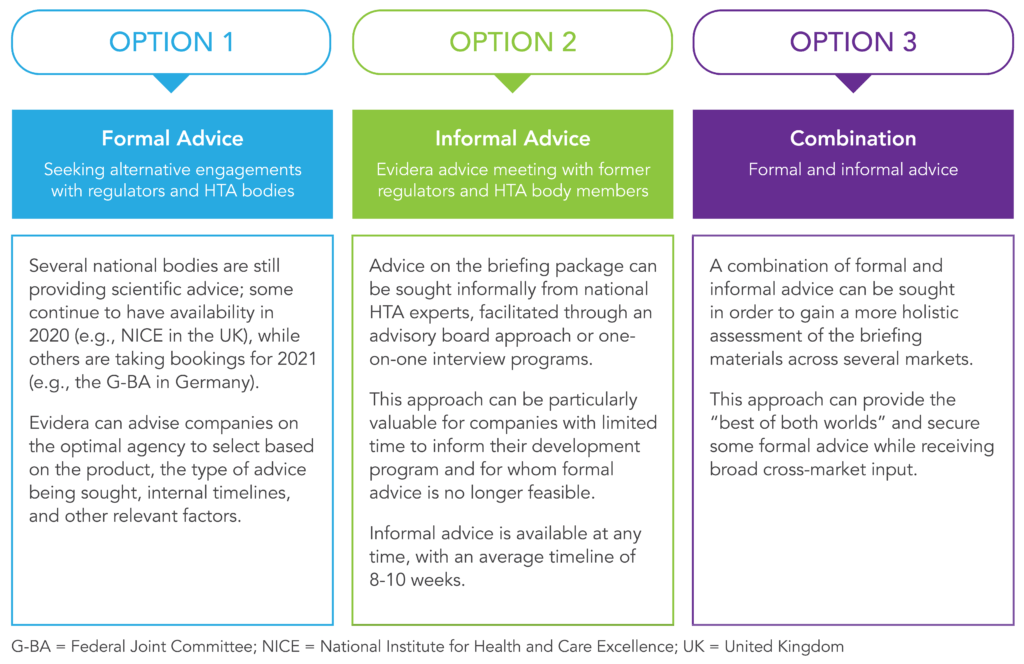
Evidera is closely monitoring the impacts of the COVID-19 pandemic on scientific advice procedures and can advise and support companies in navigating the evolving landscape. For companies seeking input into their product’s clinical development plan, there are still several options available.
References
- Correspondence with EMA, 12 May 2020
- Public domain
- Correspondence with EUnetHTA, 11 May 2020
- Internal insight
- Correspondence with CADTH, 6 May 2020
- Correspondence with NICE, 5 May 2020
- Correspondence with HAS, 7 April 2020
- Correspondence with G-BA, 6 May 2020
- Correspondence with AEMPS, 4 May 2020
For more information, please contact us