Join Us at ISPOR Europe
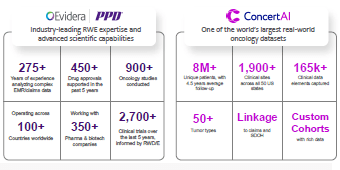
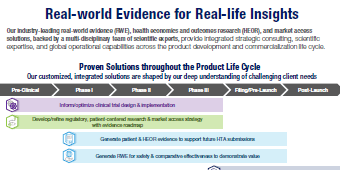
Connect with Evidera and CorEvitas experts specializing in health economics and outcomes research (HEOR), health economics and market access (HEMA), patient-centered research (PCR), and real-world data (RWD) and evidence (RWE). Our multi-disciplinary team was purpose-built to provide integrated solutions across the product development and commercialization lifecycle. Our deep expertise and proprietary real-world data offerings are designed to simplify your complex research needs, providing seamless support from initial development to market access.
Visit us at Booth # 901 or schedule a meeting on-site to discuss our customized offerings.
Are you ready for the EU HTA Regulation changes?
Book a free consultation at ISPOR EU with our health economic and value access experts to discuss the complex nuances of the evolving EU market access landscape. Let our experts help you navigate the implications of the new regulation to uncover potential hurdles in your HTA strategy.
Request a meetingPrivate Workshops at ISPOR EU – RSVP to attend
Our Solutions
Which patient engagement strategies will optimize your clinical study?
Can our real-world data and evidence solutions fast-track approval and secure market access for your asset?
Are you ready for the new EU HTA Regulation changes?
HEOR Theatre Presentation
Tuesday, 19 November • 3:45pm – 4:15pm
Poster and Exhibit Hall, 021, Level P0, Theater 2
Better and Faster: Automating Model Building and Statistical Analyses
Presenters: Jack Ishak, Apoorva Ambavana
Moderator: Agnes Benedict
ISPOR Forum
Tuesday, 19 November • 11:45am – 12:45pm
Using Health Preference Methods for Value Clarification in Patient Decision Support: Current Use and Future Developments
Speaker: Caitlin Thomas
Symposia & Workshop Sessions
Session 2
Monday, 18 November • 5:00pm – 6:00pm
How Do We Unleash the Ambition of the EU HTA Regulation through Practical Methodological Solutions?
Moderator: Martin Parkinson
Panelists: Anna Chaimani, Niklas Hedberg, James Ryan
Session 3
Tuesday, 19 November • 1:45pm – 2:45pm
How Can We Move from Generating Robust Patient Preference Information to Producing Decision-Ready Outputs?
Discussion Leader: Sebastian Heidenreich
Discussants: Paul Schneider, Divya Mohan
Session 4
Tuesday, 19 November • 5:00pm – 6:00pm
Interplay Between Budget Impact Analysis (BIA) and Cost-Effectiveness Analysis (CEA); Theory vs Practice
Moderator: Cornelis Boersma
Panelists: Jaime Caro, Josephine Mauskopf, Thea van Asselt
Session 5
Wednesday, 20 November • 10:00am – 11:00am
Taking the “Greener” Pill – a Case Study for Incorporating Carbon Footprint in Health Technology Assessment
Discussion Leader: Ruth Chapman
Discussants: Michael Cohen, Grace Hampson, Kinga Marczell
Poster Presentations
Session 1
Monday, 18 November
Phil Leventhal, Danielle Drachmann, Rienne Schinner, Soren Skovlund
Louis Matza, Katelyn Cutts, Karen Malley, Karin Coyne
Louis Matza, Katie Stewart
Carolina Casañas Comabella, Phil Sarocco
Keila Meginnis
Danielle Rodriguez, Carla Dias-Barbosa, Karen Bailey, Dina Filipenko
Naomi Stapleton, Heather Burnett, Jennifer Knight
Caoimhe Rice, Sara Carvalho, Jennifer Davidson
Caoimhe Rice, Jennifer Davidson
Session 2
Monday, 18 November
Himani Jaiswal, Paulina Rolska-Wójcik, Caroline Delaitre-Bonnin
Katie Stewart, Louis Matza
Nick Boucher
Paulina Kazmierska, George Bungey
Lu Ban
Ashwin Rai, Victoria Ikoro, Ariel Berger
Ariel Berger
Session 3
Tuesday, 19 November
Anna D’Ausilio, Rhythm Arora, Brian Connelly, Ariel Berger, Haripriya Jain
Ayumi Hamaguchi, Amanda Pulfer, Dimitra Lambrelli
Ashley Duenas, Claudine Clucas, Klaudia Kornalska, Paul Swinburn
Session 4
Tuesday, 19 November
Ayumi Hamaguchi, Amanda Pulfer, Dimitra Lambrelli
Nick Boucher, Ariel Berger
Karina Palhares
George Bungey, Jorgen Moller
Sohan Deshpande
Lalith Mittal
Gabor Szabo, Amy Pinsent, Mahmoud Slim, Shannon Sullivan, Agnes Benedict, Simone Rivolo
Jennifer Knight, Heather Burnett, Naomi Stapleton
Milena Anatchkova, Tsion Fikre
Session 5
Wednesday, 20 November
Louis Matza, Timothy Howell
Nahila Justo, Yi Lu, Kathryn Evans, Valerie Olson, David Turner
Carolina Casañas Comabella
Walter Morris, George Bungey
Lalith Mittal
Caoimhe Rice, Sara Carvalho, Jennifer Davidson
Carla Dias Barbosa, Karen Bailey
Milena Anatchkova, Tsion Fikre