JULY 2021, PERSPECTIVE PAPER
 Jonca Bull, MD Vice President Value & Development Consulting Evidera, a PPD business |  Catriona Roscoe-Cutting Executive Director Value & Development Consulting Evidera, a PPD business |
Orphan drug designation (ODD) provides several compelling incentives and benefits for pharmaceutical organizations developing drugs to treat rare diseases. Whether you are requesting the designation from U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), or another health authority, it’s an application best approached strategically. We offer four strategic considerations to bear in mind as you prepare for your ODD application.
Consideration One: Whether to Apply
If your therapy is clearly targeting a well described rare disease or condition that meets the rare population criteria, you already have clinical data in that disease with proven activity, and an elucidated mechanism of action (MOA), then your ODD application is quite likely to be successful. If there are outstanding questions however, around your scientific rationale or population estimate perhaps, or there are designations/approvals that may be perceived as competitive or blocking your program due to exclusivity, then you may benefit from a viability assessment.
ODD applications gather data from a variety of disciplines. It is therefore critical to approach this assessment with a cross-functional team of subject matter experts. Ideally, this team should include a clinician in your therapeutic area as well as experts in pharmacology, epidemiology, and regulatory affairs (nonclinical toxicology, chemistry, manufacturing, and controls [CMC] and strategic consulting). Together, the team can evaluate your data and MOA in context with the epidemiology of the disease state and therapeutic landscape to put together a holistic picture of your viability.
This assessment can get quite complex. As one example, when considering the MOA to determine what distinct disease or condition the drug is intended to treat, diagnose, or prevent, both the FDA and EMA take into account whether a medical condition constitutes a distinct disease or condition dependent on a number of factors that are assessed together in the context of the specific drug for which designation is requested. In the FDA guidance and similarly stated in EMA guidelines these would include:1
- Pathogenesis of the disease or condition
- Course of the disease or condition
- Prognosis of the disease or condition
- Resistance to treatment
In general, subsets of a condition otherwise over the population criteria, would not be considered for ODD except where that subset presents with distinct and unique evaluable characteristics that have a plausible link to the condition and such characteristics are essential for the MOA of the product.2,3 While orphan subsets are a tricky construct to navigate, at the same time, agencies do not want to create barriers to improvements in treatment, so they can be a viable strategy for some developers where appropriately supportive data exist.
The other piece of this puzzle is that an early analysis can assess the fact that not every box in an ideal application needs to be ticked to develop a successful application. It surprises some to learn that even if there are gaps in their application (e.g., perhaps there is not yet any clinical data nor relevant preclinical models), a promising MOA may be enough to grant an ODD. While the strongest data to present is clinical data—and the more, the better—if there is precedent with a drug with the same MOA or if you can explain the MOA with compelling in vivo data, you may have a strong application even before entering in-human studies. This scenario ties into the next consideration on our list.
Consideration Two: When to Apply
Analysis of ODD viability should ideally be completed at the earliest stages of development to help shape your strategy of when to apply. We have seen clients with a strong scientific rationale pursue and achieve an ODD even while, in tandem, planning their first in-human study. While this can be a high-risk strategy, achieving this milestone early in development can also potentially provide high rewards.
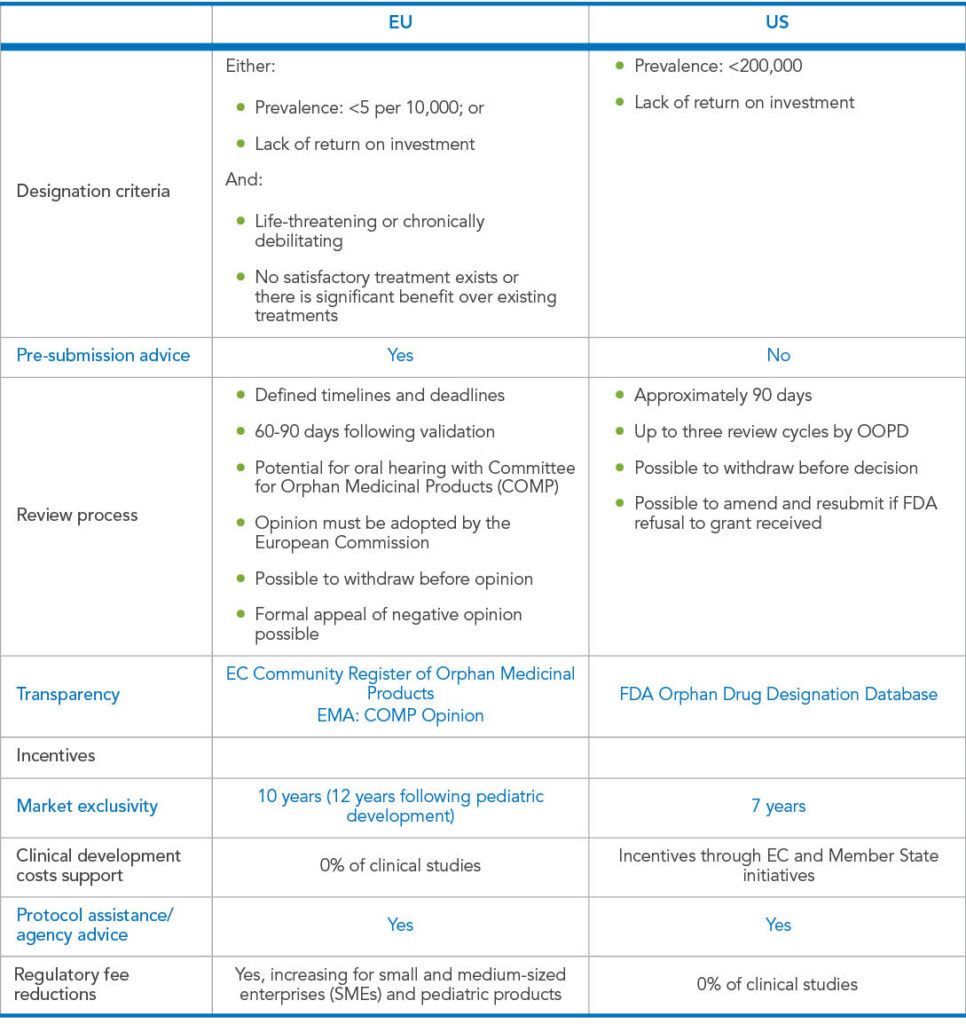
Going early presents all the expected benefits of ODD such as tax credits, market exclusivity post-approval (seven years in the US and 10 years, rising to 12 years with pediatric development, in the EU), waivers and reductions of regulatory fees in both the EU and US. An early ODD also helps jump-start a development program in very practical ways. It can lead to an increase in the stock price of a company (average by 3.36%)4 after the announcement of the designation, increasing the value for companies, particularly for small biotechs. It can substantially boost credibility while potentially attracting the attention of investigators, patient advocacy groups, investors, the media, and other important influencers.
Additionally, ODD could open the ability to qualify to compete for research grants from the FDA’s Office of Orphan Products Development (OOPD) and from the European Commission to support clinical studies for orphan drugs. For example, the FDA, recognizing that unlike with common diseases, there is little existing knowledge on the natural history of many rare diseases, developed its Orphan Products Natural History Grants Program. The program supports characterization of the natural history of rare diseases/conditions, identification of genotypic and phenotypic subpopulations and development and/or validation of clinical outcome measures, biomarkers and/or companion diagnostics. This can address a common barrier in rare disease development: if you don’t have a baseline of how a disease progresses without treatment, it is difficult to determine the effect of the treatment.
Natural history also ties into the population estimate section of the ODD application, a critical section. Applications are sometimes denied because of not meeting rare disease prevalence requirements (i.e., trying to submit for something that is not well supported as a rare disease or as a subset of a larger disease). Therefore, having a very experienced epidemiologist on your ODD application team is critical. Again, and we can’t emphasize this enough, the ODD application takes a full cross-functional and experienced team.
Of course, despite all the potential benefits, the pursuit of an orphan regulatory pathway early in your development program when you have less data—or even no clinical data—also carries a higher risk of denial. While a negative decision can still be appealed, it is important to begin with a sound submission strategy after completing an ODD due diligence analysis to mitigate this risk as much as possible. One such submission strategy is outlined in the next consideration below.
Consideration Three: With Whom to Apply
Encouraged by the success of the FDA/Office for Orphan Product Development (OOPD), regulatory procedures and financial incentives have been implemented worldwide to encourage the development of orphan drugs. In 1993, Japan adopted similar measures to the FDA, conferring 10 years’ regulatory exclusivity to orphan drugs. Australia followed next in 1997 with a 5-year exclusivity regulation. In 2000, the European Union followed with regulation that, like Japan, provides 10 years’ exclusivity5, extending a further two years where a Pediatric Investigational Plan (PIP) is completed.6
Pharmaceutical developers may be hesitant to reach out to regulatory offices, but in our experience, both the FDA and EMA recognize that they may be working with companies that require some help throughout the process, and tend to be quite open to questions and engagement. Interaction with health authorities, in turn, can provide valuable input around the development at the earliest stages to help validate the promise of the therapy.
Overall, the ODD application process and requirement between the EMA and the FDA is quite similar. However, each regulatory agency has their own processes, timelines, specific definitions, and criteria for meeting the epidemiology threshold. One benefit of the EMA pathway includes the unique option of a free pre-submission consultation by teleconference, offering an invaluable opportunity to get in-depth scientific consultation on the viability of your draft application and insights on modifications and additional data that may be needed for a successful application.7
For this reason, it may be a good strategic consideration for developers seeking global development to initiate the ODD process with the EMA and leverage their feedback when applying to the FDA.
On the other hand, EMA requires either lack of satisfactory treatments or data supporting a justification for designation based on the potential for significant benefit over existing treatments to earn ODD, while the FDA’s requirement of a positive benefit-risk assessment is at a lower threshold, considering these to be requirements for orphan market exclusivity rather than for designation.
Consideration Four: How to Apply
Both the FDA and EMA offer a wealth of recommendations and guidance documents on their web sites to help navigate the details of their application process. The key is to identify the core data that supports the orphan designation and to write a well-reasoned, well-crafted, and succinct application. Health authorities aren’t looking for 200 pages. In fact, they are satisfied if the application is 20-30 pages as long as the scientific rationale is clear, the disease or condition is well defined, and you provide a determination of the population estimate that supports that the disease is rare. Information should also be referenced in full, allowing reviewers to readily track and check all claims.
Key elements of the application include the following with the most critical sections in bold:
- An overview of the rare disease, current treatment options and rationale for the investigational product’s (IP) development in this area of high unmet medical need.
- The scientific rationale for the IP in the rare disease describing the MOA and all relevant and available in vitro, in vivo, and clinical data.
- Details on the regulatory and marketing status of the IP.
- Documentation on the epidemiology of the rare disease using appropriate data sources, methodologies, and calculations.
- In the EU, a discussion of significant benefit over current treatment options.
References
- U.S. Food and Drug Administration. Orphan drug designation: disease considerations. Published online May 10, 2019. Accessed June 7, 2021. https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/orphan-drug-designation-disease-considerations
- Office of Orphan Products Development (OOPD). Recommended Tips for Creating an Orphan Drug Designation Application. 2018. Accessed June 30, 2021. https://www.fda.gov/media/111762/download
- EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL. Guideline on the format and content of applications for designation as orphan medicinal products and on the transfer of designations from one sponsor to another. Accessed June 30, 2021. https://ec.europa.eu/health/sites/default/files/files/orphanmp/doc/2019_cons_guideline_appdes_en.pdf
- Miller KL. Do investors value the FDA orphan drug designation? Orphanet Journal of Rare Diseases. 2017;12(1):114. doi:10.1186/s13023-017-0665-6
- National Organization for Rare Disorders (NORD). Orphan drugs in the United States: an examination of patents and orphan drug exclusivity. Accessed June 8, 2021. https://rarediseases.org/wp-content/uploads/2021/03/NORD-Avalere-Report-2021_FNL-1.pdf
- Official Journal of the European Union. on medicinal products for paediatric use and amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1901&from=EN
- European Medicines Agency. Applying for orphan designation. Published September 17, 2018. Accessed June 9, 2021. https://www.ema.europa.eu/en/human-regulatory/research-development/orphan-designation/applying-orphan-designation