Challenge 1
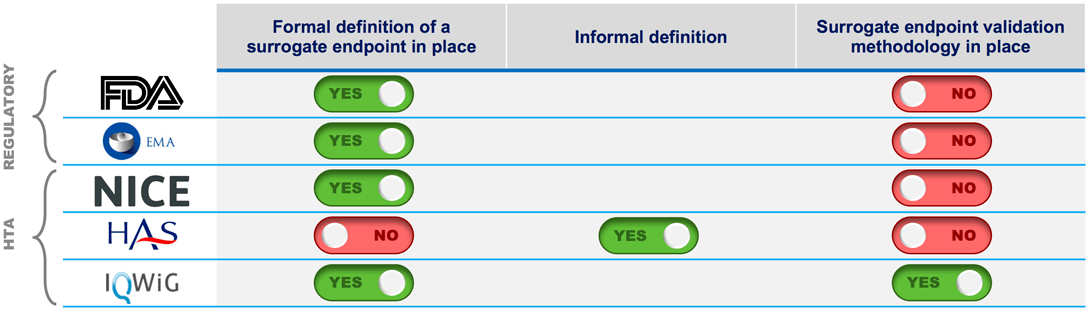
Defining a surrogate endpoint is one side of the coin, the validation is the other. Where do regulators and HTAs stand on validation?
The FDA and EMA will often accept evidence from clinical trials that shows a clear benefit on a surrogate marker, subject to an acceptable benefit/risk ratio for patients. Payers, however, consistently seek a surrogate endpoint to be patient-relevant with a strong correlation to a hard clinical endpoint.
Challenge 2
Across markets, there are different levels of payer confidence using evidence based on outcomes from surrogate endpoints in value substantiation, and different levels of payer expectations on how to address uncertainties in value demonstration.
See what payers had to say:
In a survey executed with payers in selected markets, German payers expressed a total lack of confidence when using results from surrogate endpoints in patient benefit assessments, and in most cases, require a formal validation. U.S. payers are not fully confident when considering surrogate endpoints and require the manufacturer to show evidence of the correlation to a patient-centered outcome.

SE: Surrogate endpoints; QoL: Quality of life; QALY: Quality-adjusted life years; P+R – Pricing and Reimbursement * Based on Evidera study with 6 national payers per market

U.S. payers want to see correlation of the surrogate endpoint to a patient-centered outcome (e.g., survival, QoL, functionality, pain reduction) and strong emphasis on patients’ perspective to inform clinical relevance when using a surrogate endpoint in P&R evaluations.
Traditionally, the biggest emphasis on surrogates has been in oncology. The issue is always that there are not enough patients and not enough time to have one of the patient-centered endpoints such as overall survival and QoL. These endpoints are what patients are most interested in.

NICE will look to the following factors to inform clinical relevance of a surrogate: Evidence of correlation to the final clinical endpoint (e.g., validation studies); evidence of other markers ‘that point in the same direction as the surrogate’; reported patient relevance of the surrogate endpoint (e.g., from patient organizations).
I am concerned because I think they have been used as a short cut to achieving regulatory approval and market access in situations where clinical data collection is feasible and necessary to understand the full incremental clinical value/detriments vs. current standard of care.

IQWiG/GBA will require a surrogate endpoint to be validated within a full validation study (i.e., collection of the surrogate endpoint and a hard clinical endpoint within the same study, and comparison of the correlation).
It’s about whether the endpoint is patient relevant or not. When something is patient relevant then it can be a surrogate endpoint. I am concerned about the inappropriate use of surrogate endpoints (ethically there must be a clear reason not to collect hard clinical endpoints.)
Challenge 3
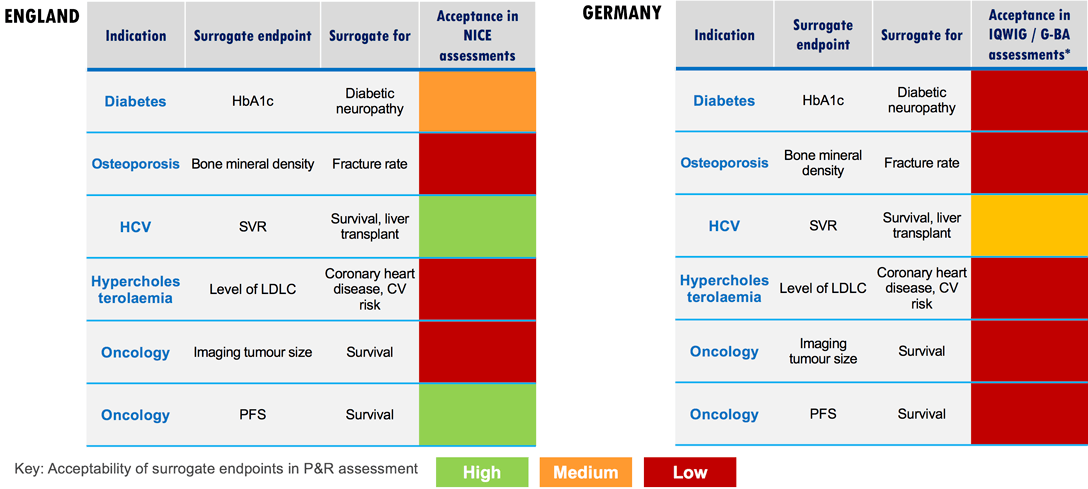
Within markets and across indications, there are different levels of payer willingness to accept surrogate endpoints in value assessments.
Read more about surrogate endpoint validation and see examples of surrogate endpoint acceptance by indication and country:
In principle, the use of surrogate endpoints is considered acceptable if validated. However, comprehensive data are required to validate a surrogate endpoint, preferably a meta-analysis of several randomized trials showing sufficient certainty of results. In order to demonstrate validity, a high correlation between effects on the surrogate and the patient-relevant endpoint is usually required (0.9 is considered as a potential threshold).
In cases where no high correlation is evident, surrogate threshold effect (STE) can be provided to support the validity if sufficiently large effects on the surrogate have been shown. It is not readily possible to transfer conclusions about the validity of surrogates across different diseases, disease grades, or interventions.
Challenge 4
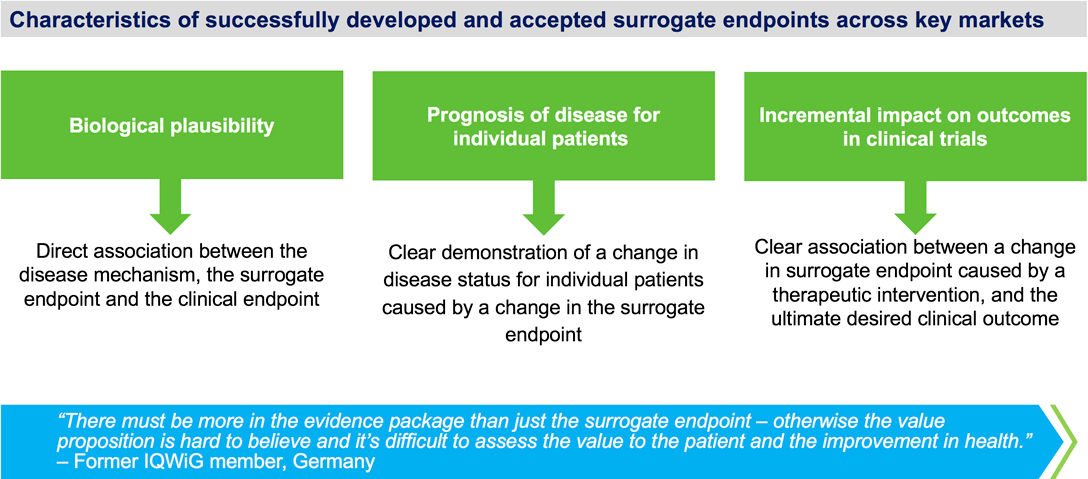
Effective use of a surrogate endpoint to support payer and HTA value assessments requires a clear chain of evidence linking a change in the surrogate parameter with a change in clinical outcomes.
When is a surrogate endpoint most likely to be an appropriate/acceptable endpoint for a pivotal clinical trial?
A surrogate endpoint is most likely to be an appropriate/acceptable endpoint for a pivotal clinical trial if the following factors are in place.
- A clear and transparent rationale as to why it is not feasible to collect hard clinical endpoint data, for example, when a requirement for a long follow-up is not feasible within a clinical development program (especially important for innovative drugs where there are few alternatives and, therefore, there may be more pressure to make the drug available)
- All criteria for the validity of a surrogate endpoint are met, including:Consistency of the association between the surrogate and clinical endpoint
Consistency of the association between surrogate endpoints and patient important outcomes (e.g., quality of life (Qol), pain reduction, activities of daily living) - Evidence from trials in the same drug class that improvement in the surrogate endpoint has consistently led to improvement in the target outcome and evidence from trials in other drug classes that improvement in the surrogate endpoint has consistently led to improvement in patient important outcomes
Do You Need Further Insight Into Using Surrogate Endpoints?Evidera’s Market Access Consulting Team Can Help.


