Now, more than ever, it is critical to have a global partner with an in-depth understanding of the new regulation and its complex nuances. The right partner will provide you with value and access consulting to help you navigate this major change to the EU market access landscape and address the following concerns:
- Determining whether there is a need to identify a new evidence strategy considering the new regulation
- Learning how to equip your organization to robustly prepare for this major change
- Implementing the optimal Integrated Scientific Advice strategy with regulatory and HTA for your asset amid this major change
- Understanding the value of Joint Scientific Consultation (JSC), determining if it is necessary for your product, and if so, when and how to initiate and implement it
- Understanding which considerations will be given to real-world evidence (RWE) in the evaluation process
- Handling the challenge of member states being allowed an unlimited number of population, intervention, comparison and outcomes (PICO) elements
- Interpreting the role of patients and healthcare professionals in Joint Clinical Assessments (JCAs)

In a challenging CGT market access environment, learn five key considerations to navigate the new HTA.
Download our white paperMove forward in uncertain regulatory times
The full consequences of what the regulation means to pharmaceutical and technology developers remains unseen. At Evidera, our experts have unparalleled experience with HTA submissions and joint scientific consultations. We are actively working with companies to prepare strategies and evidence plans to ensure pharmaceutical and technology developers are equipped for the new process. Whether you have one asset or a full portfolio, our team is strongly positioned to help you navigate the changes ahead.
Our team is closely tracking all HTA guidance documents, comments from public consultations and the first JCA reports to glean insights that can help shape your plan forward. With planning, early engagement and ongoing communication with HTA bodies, we can help you gain insights to drive critical decisions needed to successfully move your product forward.
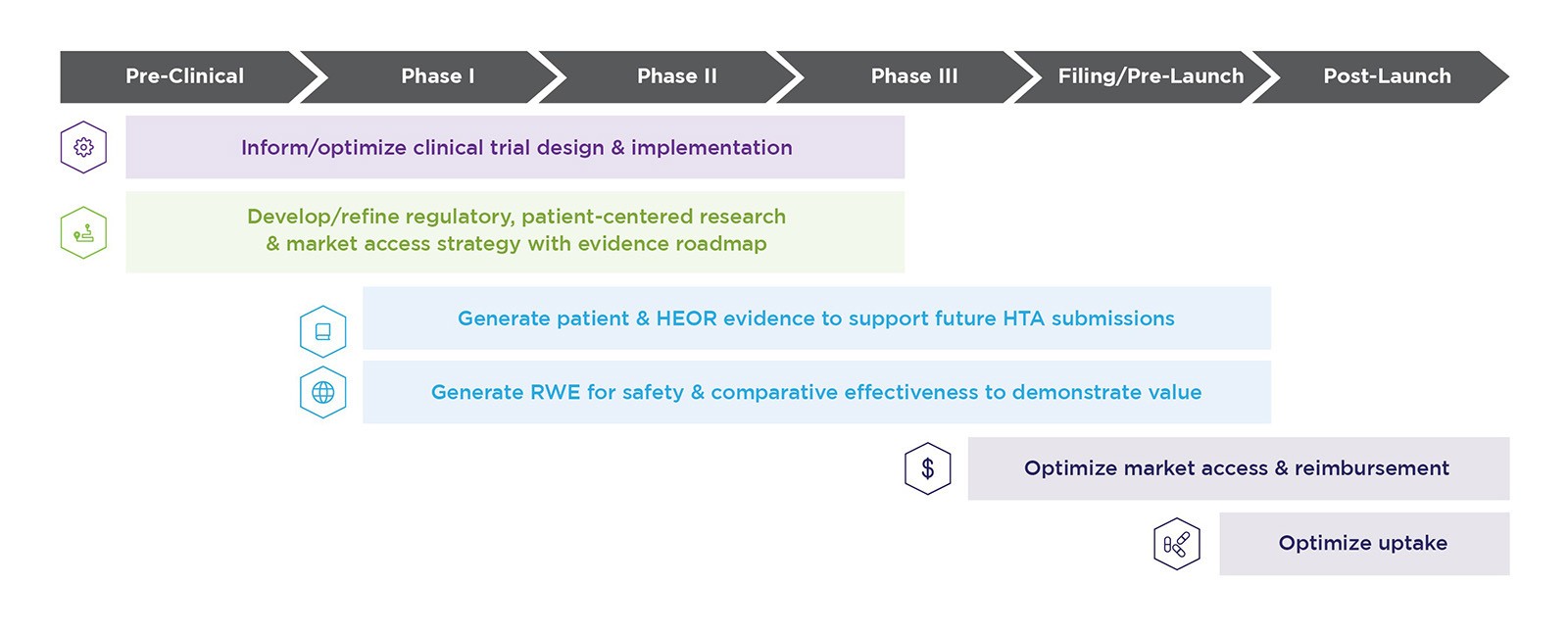
Successful preparation and implementation includes solutions across the life cycle. When you partner with Evidera, you can expect:

Engagement with regulators and HTA bodies
Through our Integrated Scientific Advice, we ensure alignment on evidence needs, identify challenges and seek regulatory and HTA guidance — allowing us to move your programs forward with speed and agility.
Identification of critical evidence to stay ahead of changing global payer/HTA demands
From health economic models to support cost-effectiveness, to pricing strategies and innovative value demonstration and delivery, our market access experts provide actionable insights to demonstrate product value and optimize reimbursement.
Industry-leading experts in early development planning and asset optimization
We generate robust plans relevant to all stakeholders that maximize trial efficiency, leverage real-world evidence (RWE) and satisfy payers.