FALL 2020, THE EVIDENCE FORUM, WHITE PAPER
 Mariah Baltezegar, MBA Executive Director, Head Peri- and Post-Approval Study Innovations Real-World Evidence Evidera, a PPD business |  Eva Kewitsch, PhD Senior Project Manager Peri- and Post-Approval Studies Real-World Evidence Evidera, a PPD business |  Kristin Kluthe, PMP Director Project Management Peri- and Post-Approval Studies Real-World Evidence Evidera, a PPD business |
COVID-19 has significantly impacted the way we live, work, and play. It has also had a significant impact on clinical care and real-world research. When the pandemic began, routine clinical care was largely put on hold and then shifted to virtual visits when feasible. The same can be said of clinical research for medical products. While some pharma companies opted to put their studies on hold and others kept studies open, the pandemic’s impact has been notable.
According to Global Data,1 69.9% of clinical trials were interrupted because of enrollment suspension. Results from a survey of over 5,000 studies and 198,000 study sites globally2 showed a decline of 59% in new patients entering study sites as of April 2020 compared to 2019 levels, with a decrease in that decline to 20% in August 2020. This same study showed the administration of study drugs has been interrupted due to the inability of patients to access sites and the pandemic’s effect on study drug supplies. These interruptions stem from concern for patient safety, lack of staff, and site access for medication administration as some medications must be given in a healthcare facility. Before COVID-19, perceived or actual regulatory or ethics committee hurdles and lack of willingness to try something novel kept the virtual clinical study model from being broadly implemented. However, the desire to continue bringing safe and effective treatments to patients, even during a global pandemic, has led to accelerated adoption of virtual study approaches; 67% of the Global Data respondents noted that COVID-19 is the reason for use of a decentralized model for the first time.1
Defining the Virtual or Decentralized Study Model
Virtual clinical studies go by many names, including decentralized and remote, but the concept is the same: bring the study directly to the patient or their caregiver. This often involves leveraging technology such as eConsent, TeleVisit, electronic clinical outcome assessments (eCOA)/electronic patient-reported outcomes (ePRO), devices and wearables, patient engagement platforms, and virtual study platforms. These virtual or remote strategies can also be supported by home health nurses and phlebotomists or direct-to-patient supplies. This allows the patient to take part in the study from their home, office, or on the go.
Putting these enablement approaches together requires careful deliberation. There are many aspects to consider when assessing the fit of a study to a virtual approach beyond just capability. This includes:
- The geographic areas where the study will be conducted
- Where and how patients will be recruited
- Where follow-up will be performed
- Whether follow-up requires clinical (physician) review
- If the study data is required for registration or regulatory purposes
- Whether electronic informed consent (eConsent) can be used
- Whether the report of measures can be completed by the patient/caregiver and if clinician confirmation is required
Once these questions have been answered, an assessment of the appropriate technology partner and other strategies to support virtualization is undertaken. For technology, it’s important to consider:
- Whether the platform complies with regulatory requirements (if required)
- Data protection regulations
- If devices must be provisioned or whether study assessments can use the Bring Your Own Device (BYOD) approach
- Whether the platform supports multiple languages
- If the user interface is intuitive
Determining a Good Fit for a Virtual Model
Many study types are a good fit for a virtual model. It can be related to the indication or the phase but more specifically about the study approach and types of data that need to be collected. When designing studies to fit the virtual model it is important to build a flexible protocol from the start that will allow for remote capture of study data, where appropriate. There are some things to consider when deciding on a virtual approach:
- Ideally no equipment should be needed, or if required, portable equipment should be available to perform protocol-required assessments
- Geographic footprint includes countries where regulatory, ethics, and cultural norms allow for remote collection of data
- Patient recruitment can be accomplished electronically or is not needed
- Patient population motor and cognitive functions are at the levels that they can enter data digitally via apps or devices
- Drug, if required, is easily administered in a home setting
Case Study 1
Long-term, Post Treatment Follow-up
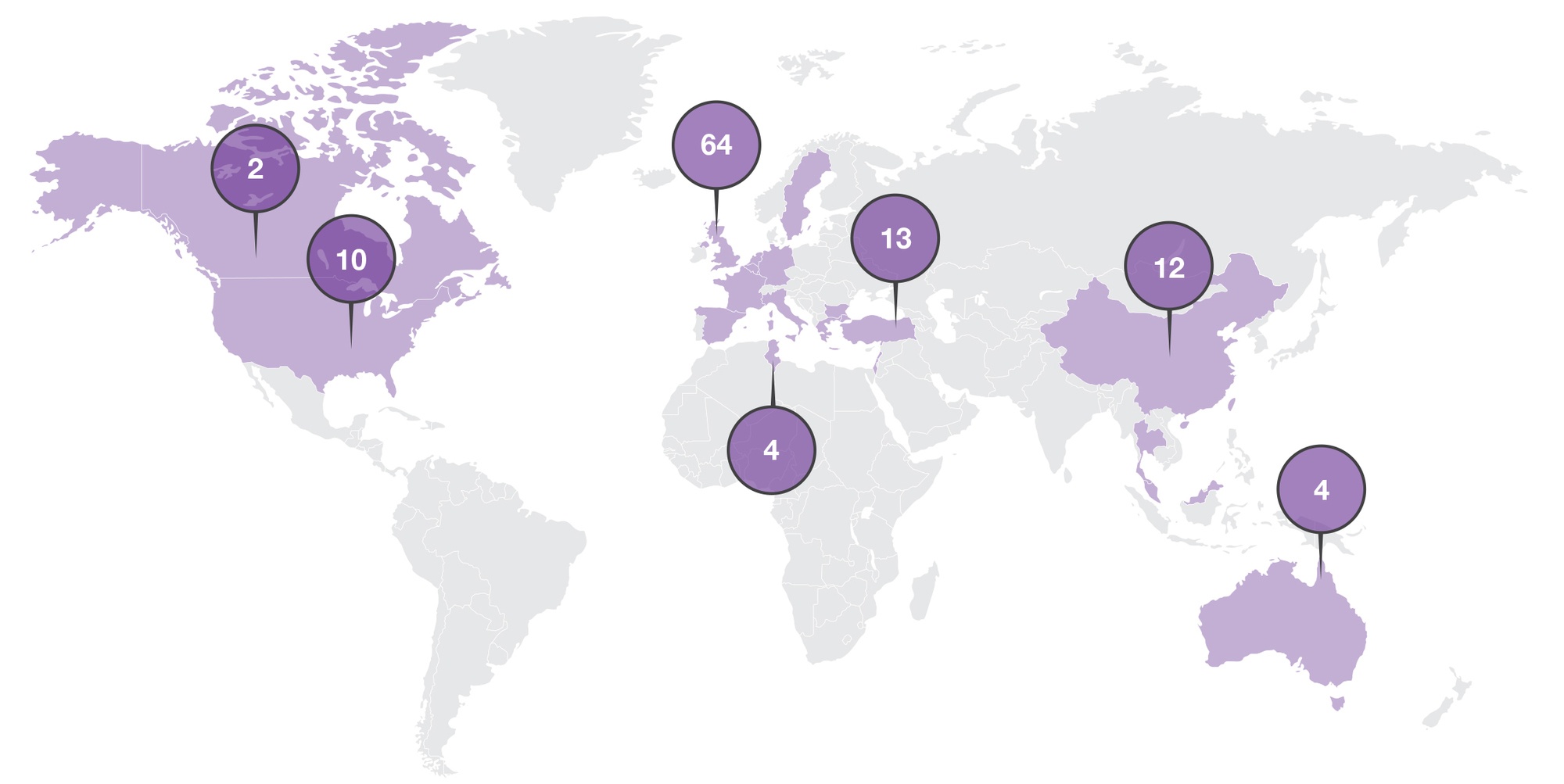
In this long-term follow-up study, patients rolled over from various parent protocols to continue treatment and, finally, long-term follow-up for several years post last dose. All assessments, except vital signs and hematology, were completed per local standard of care (SOC). Data collection for patients in long-term post treatment follow-up was minimal (only every 6 months). The study included patients from sites in the United States (US), Europe, the Middle East, and Asia (See Figure 1). As an open-label rollover study, this seemed to be a good fit for virtualization.
Virtual Approach Benefits
Virtualization for this study could result in a reduced site footprint with consolidation from multiple sites per country to a single site per country. Further benefits could include reduced burden to the patients by leveraging direct‑to‑patient data collection.
Reducing the Site Footprint
Using a virtual approach, only one central site per country may be required. All other sites would transfer the study-related activities to this central investigator for further study follow-up. In assessing the fit of this strategy for this ongoing study, we evaluated all study stakeholders when determining the potential to reduce the number of brick and mortar site locations including the patients, existing investigators, primary care providers, and the sponsor. From the patient perspective, engaging with a physician they are not familiar with could impact their willingness to continue with study-specific activities. However, given
that most of the data are SOC data collected from the primary care providers by the central site study team, there would be minimal contact with the central site principal investigator (PI), so this challenge is likely minimal.
From an existing study site perspective, closing existing sites and having all patients followed centrally by one site per country may impact the relationship between the sponsor and the PI. Also, in this study part of the patient population is on treatment and patients are transfusion dependent. As a result, on-site visits are still needed as part of SOC and benefits of site consolidation may not be realized.
Regulatory Acceptability
Given the geographic expanse of this study, there are many regulatory bodies, ethics committees, and cultural norms to consider.
For this study, remote consenting was implemented to allow consenting of long-term follow-up patients without need for an on-site visit. However, only select countries allowed this remote approach due to local regulations. This resulted in the need for a hybrid consenting approach. Using this assessment as a surrogate, it is possible that a virtual approach may not be feasible globally.
Patient Population
With most of the patient population in some parent studies being more than 80 years of age, there may also be limited capability or willingness for the patients to participate using digital tools. Subjects typically must have a smartphone, a strong internet connection, and fluency in mobile technology. These requirements, coupled with lack of in-person support, may exclude older patients.
Final Assessment
Considering all factors, this ongoing study, albeit simple in design, is not a good fit for a virtual approach. In assessing the virtual fit for this study, we identified several challenges in application of the virtual approach, including:
- Varied regulatory acceptability of approach in the expansive global footprint
- In-person visits are required for patients receiving the sub-cutaneous investigational product
- The protocol allows for either in-person or phone visits for long-term, post treatment follow-up
- Majority of patients are transfusion-dependent and need to go on-site for in-person visits for those SOC transfusions
- The average patient population is > 80 years of age for some parent studies
- There is a close relationship between patients and the site as investigators are patients’ primary specialized provider
- Multiple studies feed into this protocol over time
Case Study 2
Early Access Program for Breast Cancer Patients During COVID-19
During the COVID-19 pandemic, an alternative solution to on-site visits for an early access program was required. The solution had to be rapidly deployed to speed access to an investigational treatment for breast cancer patients. This was critically important due to their vulnerable health status and risk factors related to COVID-19. To address this need, the sponsor wanted to bring the study to the patient virtually.
Assessing Virtual Fit
This study implemented a novel approach to allow patients to continue treatment and ensure patient safety during the pandemic. The goal was to bring the study directly to the patient thereby avoiding risk of exposure to COVID-19 at the treatment clinic. Protocol-defined study procedures were developed to allow assessments to be completed in the patient’s home. This included remote sample collection, disease assessment, and study drug administration.
Enabling a Virtual Approach
In order to operationalize the direct-to-patient approach, the protocol was designed to allow for remote study visits. One of our partners in virtual study execution was selected to perform virtual site services using remote study coordinators and mobile nurses to facilitate in-home patient visits, data collection, direct-to-patient clinical supplies, and study drug administration. A single platform was implemented to digitally enable a variety of study activities including:
- eConsent
- eSource
- Electronic data capture (EDC)
- Electronic patient-reported outcomes (ePRO)
- Video telemedicine
This virtual approach worked well for this study. The study team was able to implement the approach quickly and this solution allowed oncology patients to safely receive access to an investigational product at home during the COVID-19 pandemic while allowing continuity of care. Telemedicine enabled oversight by the patient’s treating physician, which assured patient safety and gave comfort to the patients during this new treatment approach. Patients were able to receive treatments as safely as possible through a virtual approach and a well-planned, risk mitigation strategy.
Conclusion
Adjustments to old approaches and newer, innovative approaches to clinical study data collection have been born out of necessity to address research continuity during COVID-19. Key innovative approaches include digital enablement (using digital technologies to enhance the efficiency or ability to collect data remotely in a study) and virtual/decentralized studies (moving away from the site-based study model to a model where patients are the primary focus). While there are macro considerations to assess fit for these types of innovative approaches, not all studies are the right fit, and each must be assessed by its own merit. In the case studies we assessed an ongoing study for transition to a virtual study, which was not the right fit for the virtual model. We also assessed a new early access study that was a good fit for the virtual study model. When determining whether a digital or virtual model is appropriate, all individuals invested in the success of the strategy should be consulted, especially those well-versed in the right assessments to be made, stakeholders to include, and questions to ask.
A special thank you to Jodie Block and Vitalisa Mavilio for their contributions to this article.
References
- Clinical Trials. Update on Clinical Trials Disrupted Due to COVID-19. GlobalData Healthcare. 2020 May 14. Available at: https://www.clinicaltrialsarena.com/comment/disrupted-clinical-trials-covid-19/. Accessed September 21, 2020.
- Medidata. COVID-19 and Clinical Trials: The Medidata Perspective. Release 9.0. Available at: https://www.medidata.com/wp-content/uploads/2020/09/COVID19-Response9.0_Clinical-Trials_2020921_v2.pdf. Accessed October 8, 2020.
For more information, please contact
[email protected], [email protected], or [email protected].