FALL 2020, THE EVIDENCE FORUM, WHITE PAPER
 |
| Brendt Stier, MS Senior Director, Strategic Consulting Value & Development Consulting Evidera, a PPD business |
Introduction
It is very common for a biotech or biopharma company to change or improve existing drugs by creating a new formulation or dosage. In these cases, a new drug application (NDA) can often use the wealth of existing data, with a focus on specific new required studies.
Section 505(b)(2) of the Federal Food, Drug, and Cosmetic Act1 gives the United States Food and Drug Administration (FDA) express permission to rely on data not developed by the NDA applicant, such as full reports of safety and effectiveness. It may also rely on FDA findings of safety and effectiveness to the extent that the proposed drug product shares characteristics with the listed drug. This makes the 505(b)(2) pathway appealing as it helps avoid unnecessary duplication of studies already performed on a previously approved drug and can result in a less expensive and faster approval compared to traditional development paths such as 505(b)(1). One caveat is the applicant must demonstrate that the bridge between the proposed drug product and the listed drug is scientifically justified, which is where challenges can occur.
In this whitepaper we discuss how innovation can help develop the bridge needed for 505(b)(2) approval, compare traditional and streamlined approaches to clinical development, and discuss how to save money and time with hybrid protocol designs. We also use a case study as a real-world example of how innovative solutions to development strategies can reduce costs and timelines.
Pathways to New Drug Approval and Typical Development Studies
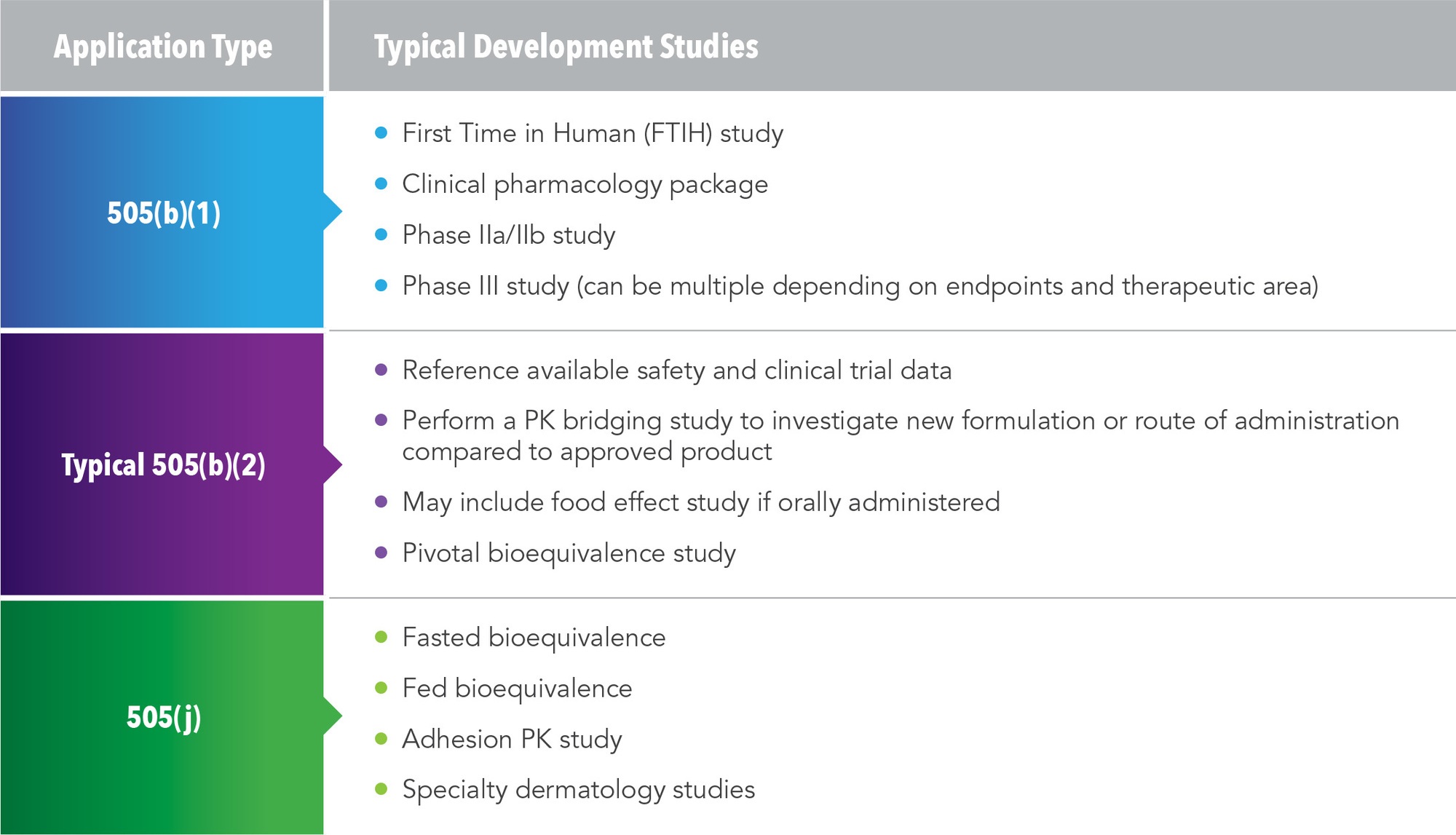
There are three possible pathways for NDAs, and each requires different development studies (See Figure 1).
505(b)(1)
This is the traditional pathway for an NDA. Studies used in this pathway include first time in human trial, clinical pharmacology package, Phase IIa/IIb studies, and Phase III studies. A traditional program under the 505(b)(1) pathway could take five years and cost several hundred million dollars.
505(b)(2)
This is an abbreviated pathway and the typical development studies are much smaller. By referencing available safety and clinical data from an approved product, a pharmacokinetic (PK) bridging study can investigate either the new formulation or new route of administration compared to the approved product and establish bioequivalence. In some cases, clinical pharmacology studies or food-effect studies will be needed. For example, an orally administered drug might need an alcohol dose-dumping study. FDA approval hinges on the pivotal bioequivalence study.
505(j)
This pathway is used for drugs that are identical to a referenced listed drug (RLD). Studies employed in this pathway include a fasted bioequivalence study, fed bioequivalence, adhesion studies, and specific dermatological studies.
Bringing Innovation to 505(b)(2)
Receiving approval through 505(b)(2) requires not just a detailed process but a 30,000-foot view of the landscape. Drug manufacturers tend to focus solely on the approval process and don’t see what other studies may be needed for their development pathway and how they can differentiate their product from what is already approved. This is where a comprehensive strategy that looks at what data is available and how to obtain the most robust data from the outset can play an effective role in achieving approval while reducing timelines and costs.
In reviewing the 505(b)(2) NDA approvals for 2019, 64 drugs were approved, 45% of which were new formulations or new manufacturers, and 25% involved a new dosage form.2 The balance are new combinations (8%), new molecular entities (8%), new active ingredients (6%), unknown (6%), or already marketed (2%). The challenge for drug developers is referencing the right existing studies and conducting the new studies needed to show bioequivalence with the already approved drug.
Changing a formulation or dosage can create some challenges to the 505(b)(2) pathway. A five-year retrospective analysis of drugs approved via the 505(b)(2) pathway showed over 80% of new dosage forms or new formulations included a bioavailability/bioequivalence (BA/BE) study and nearly 100% included food-effect (FE) studies.3 Alcohol dose-dumping studies were included in 72% of extended-release formulation NDAs.3 Older drugs that are repurposed or changed may not have had proper clinical pharmacology (i.e., drug-drug interaction (DDI) or FE) studies at the time of approval; therefore, FE or DDI studies may need to be completed. While the clinical-pharmaceutical packages may still be relevant, trials will need to be performed if they are not referenced in literature. The biggest challenge is going back and finding the information in the literature; if the information cannot be found, and therefore referenced, it needs to be created. That is where innovation comes into play.
Looking at the process of NDAs from start to finish, integrating innovative thinking into the assessment process early, and continuing that perspective through the strategy and execution stages, can have a direct impact on your program from the beginning to the end. (See Figure 2).
Figure 2. Integration of Innovation Throughout the NDA Process
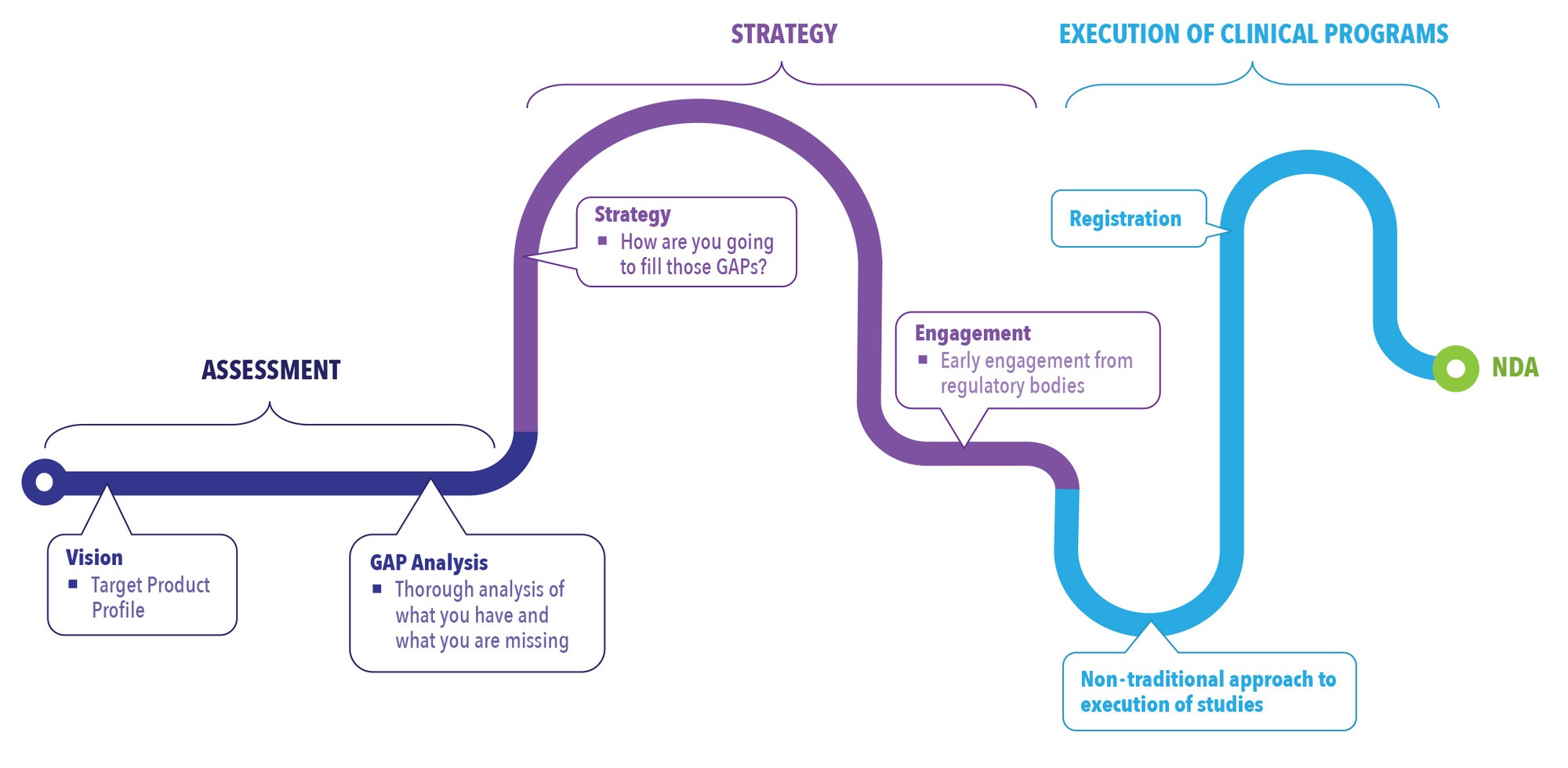
Assessment
Due diligence in the assessment stage will pay off in the long run. In this stage, a vision for the product is established through the creation of a target product profile (TPP). The patient population for the drug is identified as well as how it will be differentiated from other drugs on the market. Differentiation is not only key to marketing the drug but to developing the drug. In the assessment stage, it is important to identify how to fill the gaps that are missing from what is available on the market. A robust gap analysis established early on provides a roadmap for moving forward with development of the drug. Without this step, companies can become focused on getting the drug into clinical trials without recognizing they need to augment the available referenced data with new data that may be needed to fill in the development gaps of the program.
Strategy
Forming a comprehensive strategy utilizing available information, new information, and innovative study designs can help expedite development timelines. The strategy phase is used to determine how the gaps identified in the assessment phase will be filled. This might be through bridging toxicology, PK studies, or clinical studies. Pulling all this information together in a very comprehensive strategy early on is key to success in the 505(b)(2) pathway.
One step in the strategy phase that many companies miss is taking advantage of engagement with the FDA. A pre-investigational new drug (Pre-IND) meeting is a way to engage with the FDA early and mitigate risk. Regulatory bodies are open to innovative approaches as long the rationale in the development plans can be justified. The Pre-IND meeting should be used to lay out the strategy and seek agency agreement of the plan to help mitigate risk. Through this process manufacturers are able to get a better understanding of what regulatory bodies are looking for in the approach. This can help drug developers avoid spending money on research that would ultimately be rejected.
Execution of Clinical Programs
In the execution phase, an innovative, streamlined approach can save money and time compared to a traditional approach. The traditional approach for clinical development is less risk averse, following a sequential path and waiting for results to be available before moving on to the next study. It is a longer development pathway, and costs tend to be higher due to the cost of each study and the fact that during wait time no revenue is generated. In essence, it is development “white space.” The more time spent developing a compound, the less time that compound is on the market generating profit.
A streamlined approach is one that can be especially advantageous for smaller companies and companies looking to get on the market quicker because the development time is shorter. In a hybrid trial, multiple studies are combined under one protocol. There is some potential for higher risk, but it can be mitigated by implementing go/no-go criteria through adaptive design and other tools.
The FDA has shown they are willing to move forward with these hybrid trials, as long as risk mitigation is well defined in the protocol. A hybrid trial is going to be less expensive than several larger trials and will take less time. By decreasing timelines in development, white space is decreased, resulting in long-term savings.
Five Elements for Streamlined Hybrid Protocol Designs
Having a well-planned protocol can help mitigate concerns among regulatory bodies and institutional review boards when executing hybrid protocols. These five elements should be included in most designs:
- Safety Review Committee: responsible for reviewing the data and making key safety decisions
- Dose escalation criteria: details if certain criteria occur, the product dose is deemed safe by a medical monitor and the medical criteria in the Safety Review Committee and the dose can be escalated
- Stopping criteria: outlines the conditions that will determine when the study will be stopped or the dosage lowered based on safety and pharmacokinetic data
- Decision tree: shows how decisions will be made
- Data analysis plan: establishes when and how the data will be reviewed and by whom, if the review will be blinded, how to maintain the blind, etc.
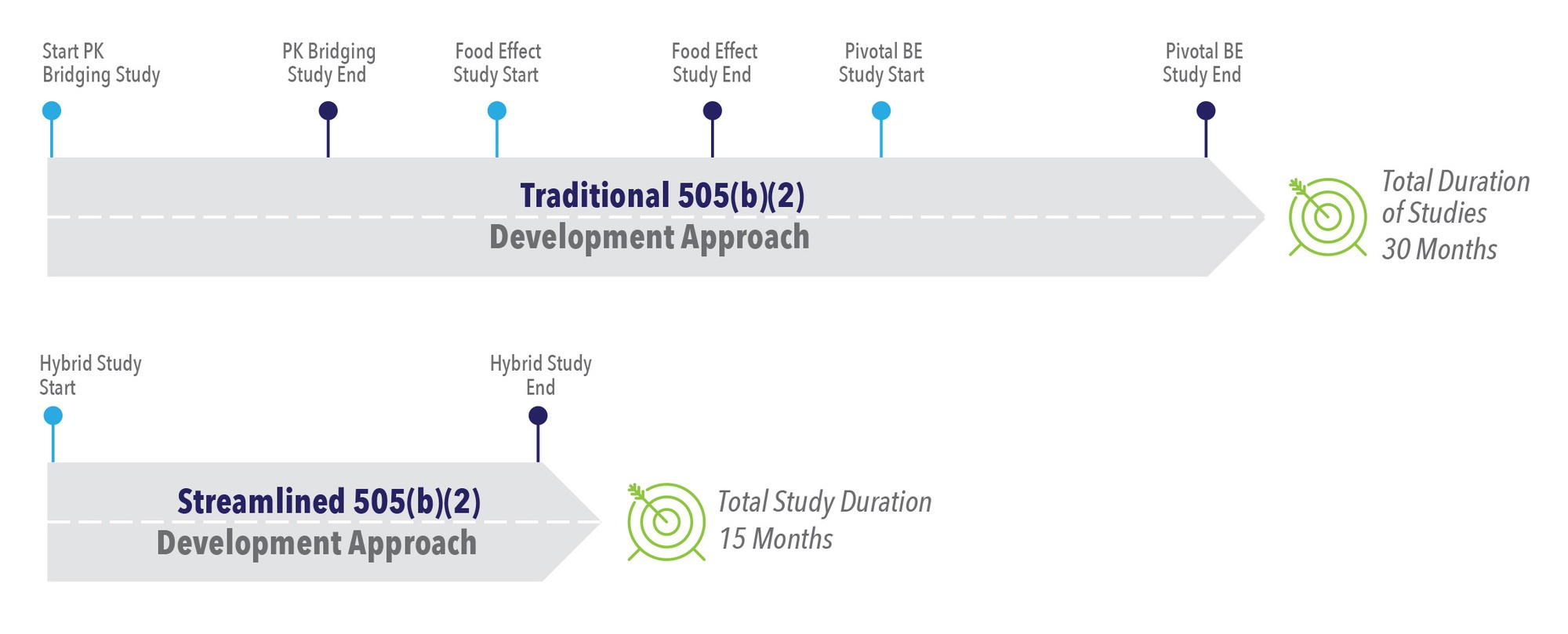
Timeline Comparison: Traditional vs. Streamlined 505(b)(2) Development
If we compare the timeline of a traditional approach with a streamlined approach for a 505(b)(2) study, the traditional approach takes approximately 30 months while the streamlined approach takes 15 months (See Figure 3). The traditional approach includes a PK bridging study that will be stopped and started, an FE study, and a pivotal BE study. In this approach, the company waits for each study to be completed before moving on to the next study. It is clearly a longer pathway.
The streamlined pathway utilizing a hybrid study combines several parts into one protocol. For example, hybrid studies can include PK bridging, FE, and pivotal BE studies simultaneously, cutting the timeline in half. This streamlined pathway has been used successfully with several clients. By running the hybrid study at one time, subjects can begin very quickly and each segment of these studies can be turned around rapidly with the analysis and interim analysis. There is no time spent waiting for separate reports and separate protocols for each study. The development timeline is shorter and costs less because it is done as one study.
Case Study: A Streamlined Approach for 505(b)(2) Development
One example to demonstrate streamlined development focuses on testing of a new formulation for a drug with better taste tolerability. Drug A was the only drug on the market for a specific disease, but the taste was awful and patients did not like taking the drug. The client was considering doing three studies: a taste assessment study with four formations to see which tasted better, a PK study to determine which formulation had the best PK profile, and a pivotal BE study to see how it compares to what was on the market.
We proposed a hybrid study instead where Part A of the study was taste assessment with a small-run PK study to see if the formulation changes the PK. If they are all the same, then Part B would take the best formulation from Part A and conduct the pivotal BE study. By utilizing a streamlined protocol, we were able to demonstrate bioequivalence and this data was used in our client’s submission to the FDA.
This approach saved the client a significant amount of time and resulted in cost savings. They were able to go to the FDA quickly, and the FDA appreciated that they were able to see each of the steps, how well they were defined, and that the criteria to move from Part A to Part B was very clear. If our client had gone with the traditional approach, they would have done three separate studies, taken at least twice as long to conduct the study, and there would have been greater costs.
Conclusion
A traditional approach is not the only way to achieve NDA approval using the 505(b)(2) pathway. Bringing innovation to all stages of the development strategy can streamline the process in the assessment stage when determining what is needed, in the strategy phase as you decide how to demonstrate the bridge between the proposed drug product and the listed drug, and in the execution of the trial. By going to the FDA early on with your plan, risk can be mitigated and the agency has shown it is willing to accept hybrid studies as long as the protocol is clearly defined, and all the components are there. In considering a 505(b)(2) pathway, taking a step back to consider all available options, including thinking outside the box, can open up novel solutions to development, ultimately leading to expedited timelines, increased time on market, and overall greater success for both patients and companies.
References
- US Food and Drug Administration. Guidance Document: Applications Covered by Section 505(b)(2). December 1999. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/applications-covered-section-505b2. Accessed October 6, 2020.
- Camargo Blog. 2019 505(b)2 NDA Approvals in Review. Available at: https://camargopharma.com/resources/blog/2019-505-b-2-nda-approvals-in-review/. Accessed September 8, 2020.
- Freije I, Lamouche S, Tanguay M. Review of Drugs Approved via the 505(b)(2) Pathway: Uncovering Drug Development Trends and Regulatory Requirements. Ther Innov Regul Sci. 2020 Jan;54(1):128-138. doi: 10.1007/s43441-019-00036-y. Epub 2020 Jan 6.
For more information, please contact
[email protected]