FALL 2020, THE EVIDENCE FORUM, WHITE PAPER
 David Nagel, MEd, MBA Executive Director, Consulting Value & Development Consulting Evidera, a PPD business |  Beth Schneider, MSc Executive Director, Site Intelligence and Activation Site and Patient Access PPD |  Stephen Powell, MBA Executive Director, Strategic Feasibility Site and Patient Access PPD |  Malcolm Horsley, BSc Senior Director, Site Intelligence and Activation Site and Patient Access PPD |  Jennifer Monen, BS Associate Director, Site Intelligence and Activation Site and Patient Access PPD |
Trends and Problem Statement
Biopharma and biotech companies make significant investments in getting their products to market. Studies are becoming more complex, driven by several factors including eligibility, endpoints, assessments, data collected, and number of sites, etc. This ever-increasing complexity in study design can frequently lead to study cost increases in excess of 25%, in addition to other implications such as difficulty in finding patients, increased effort by sites to conduct a study, more time spent in study start-up, and more costly amendments. For example, in the last two years, 73% of PPD studies have had protocol amendments between receiving the final protocol and reaching first site activated, 83% before first subject screened, and 92% before 50% of sites were activated.
While there is a high level of focus on study costs, and rightly so, the higher cost comes from study delays. One research study1 looking at lost patent days once a product was launched suggested that the impact on Expected Net Present Value (ENPV) of one protocol amendment on a typical oncology program entering Phase II or III ranged from $35 million to $75 million respectively. Given the significant cost of delays, investing the time and effort to design well thought out study protocols in initial planning will benefit companies in the long run.
What Can be Done to Avoid Lost Revenue?
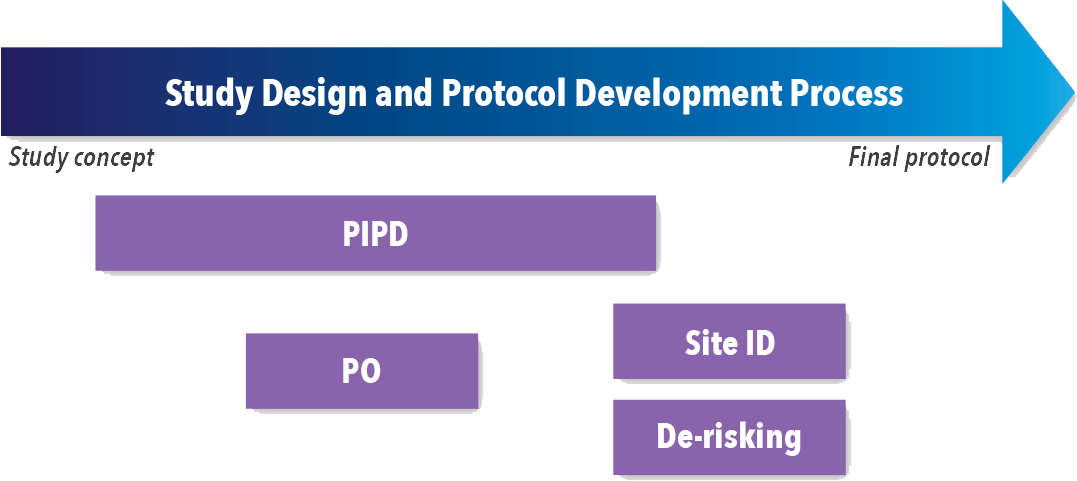
Companies should assess every study to determine the best options for reducing study complexity, cost, timelines, and amendments while designing the study. Several activities at different times throughout the study design process (See Figure 1) have shown an impact on overall success, including:
- Patient-informed protocol design (PIPD)
- Protocol optimization (PO)
- Finding the right sites (Site ID)
- Protocol de-risking
Figure 1. Timing of Study Design and Protocol Development Activities
Patient Input
PIPD refers to any form of engagement with patients to help inform elements of clinical trial study design. Common approaches include conducting patient focus groups, mock trials, patient surveys, and consulting with representatives from patient advocacy organizations. While there are many benefits of integrating PIPD into standard research practices, one key element is optimizing the study design to meet the needs of a given patient population. This has the potential to improve recruitment, patient enrollment and study outcomes; reduce patient burden and study withdrawal; ensure patients follow the assigned assessment schedules and adhere to therapy; and optimize data quality and capture, to name a few. Furthermore, involving patients during the protocol development stage is crucial to ensure the data on outcomes most relevant to patients are being collected.2
Although the quantitative impact of PIPD is still emerging, previous research has documented a 16% increase in patient enrollment3 and improved timelines, with recruitment of the first 100 patients reduced by three months.4,5
Protocol Optimization
PO can mean different things to different organizations. Here the term is used to describe a set of protocol-focused assessments meant to uncover areas where study complexity can be reduced.
When a developing protocol reaches synopsis stage, the major components of the overall draft are in place yet are still formative—making it an ideal time to step back and look for areas to optimize. A synopsis typically includes objectives/endpoints, study design/schema, number of participants, intervention groups, duration, statistical considerations, standard of care (SOC), and inclusion/exclusion (I/E) criteria. It is at this formative point that a protocol optimization assessment may help surface areas where complexity can be reduced, as well as patient burden, study cost, study duration, and likelihood of amendments.
The ISA Framework
PO requires an expert cross-functional team consisting of product development, clinical science, operational strategy, biostatistics, regulatory, and innovation. Such an expert team should be highly experienced and have access to robust data that allows for the conduct of the following assessments:
- Measure study’s site complexity and patient burden, compare to competitor studies, and recommend adjustments to the schedule of assessments; quantifying site complexity allows for better management of complexity, and several industry tools exist that allow for measurement of the complexity for a site to conduct the study
- Ensure alignment between objectives, endpoints, and assessments
- Analyze the patient eligibility criteria and recommend adjustments to increase likelihood of showing response and/or increase ability to recruit
- Evaluate standards of care, surface regional differences, and anticipate future changes
- Recommend opportunities to deploy technology to improve collection of patient-level data, reduce patient visits, and/or reduce number of sites through virtualization
- Analyze trial design and statistical methodology to recommend alternative designs or methods
- Surface alternative strategic options and the relative impact those options will have on complexity, burden, cost and time
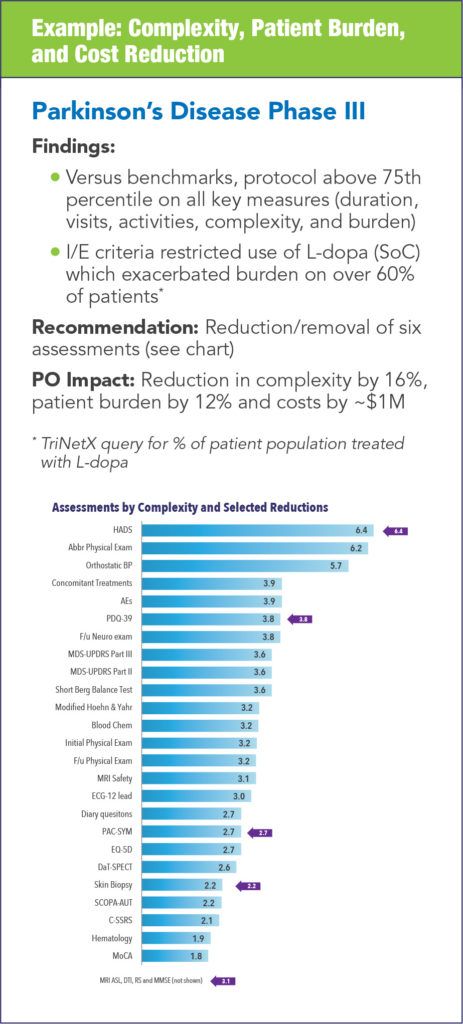
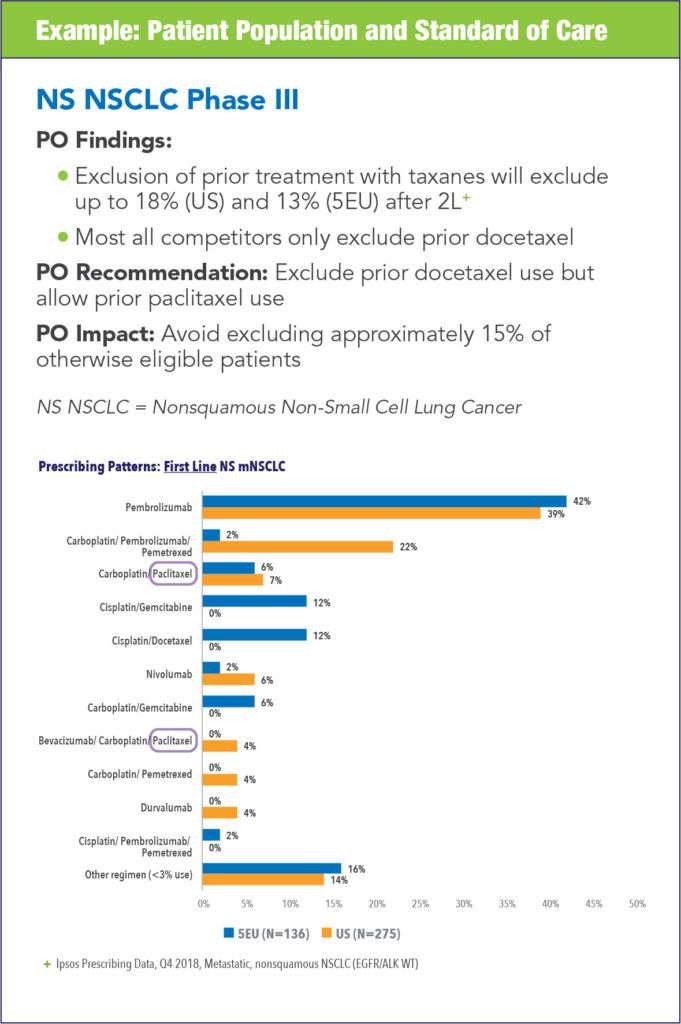
The impact of conducting protocol optimization can vary from study to study and each synopsis can yield different areas for optimization. The following examples illustrate the types of impact that can be realized.
Finding the Right Sites
Another key to successful study execution is to identify sites with the highest likelihood of success in the given indication and population. Effective use of data is essential in selecting the sites and validating their experience. There are numerous data sources in existence that can be used and more continue to become available all the time. In assessing possible sites, there is no substitute for real-world experience; therefore, the first step is to query experience data to see how sites have performed previously, in terms of enrollment, quality of data, number of monitoring issues, and speed of activation. Many contract research organizations can “score” the sites based on the parameters above to determine which are historically most successful.
There are, of course, other parameters that should be investigated. Electronic medical records (EMR) data should be examined to determine the actual, demonstrated population for the indication within the practice or institution. One example is TriNetX, a global EMR tool that allows the user to query the data to identify where patients are available. Of course, not all will qualify, but the site totals can be compared to identify which sites have larger potential patient populations. Other sources of data, such as genomics data, can be accessed to identify where patients with the specific genetic trait or marker are located in order to focus on the correct population at the correct sites.
Predictive analytics can also be used to not only show the expected activation of sites and enrollment of patients, but also to do scenario modeling as part of a protocol analysis. This can allow the formulation of alternative, potentially more effective, ways to achieve study objectives.
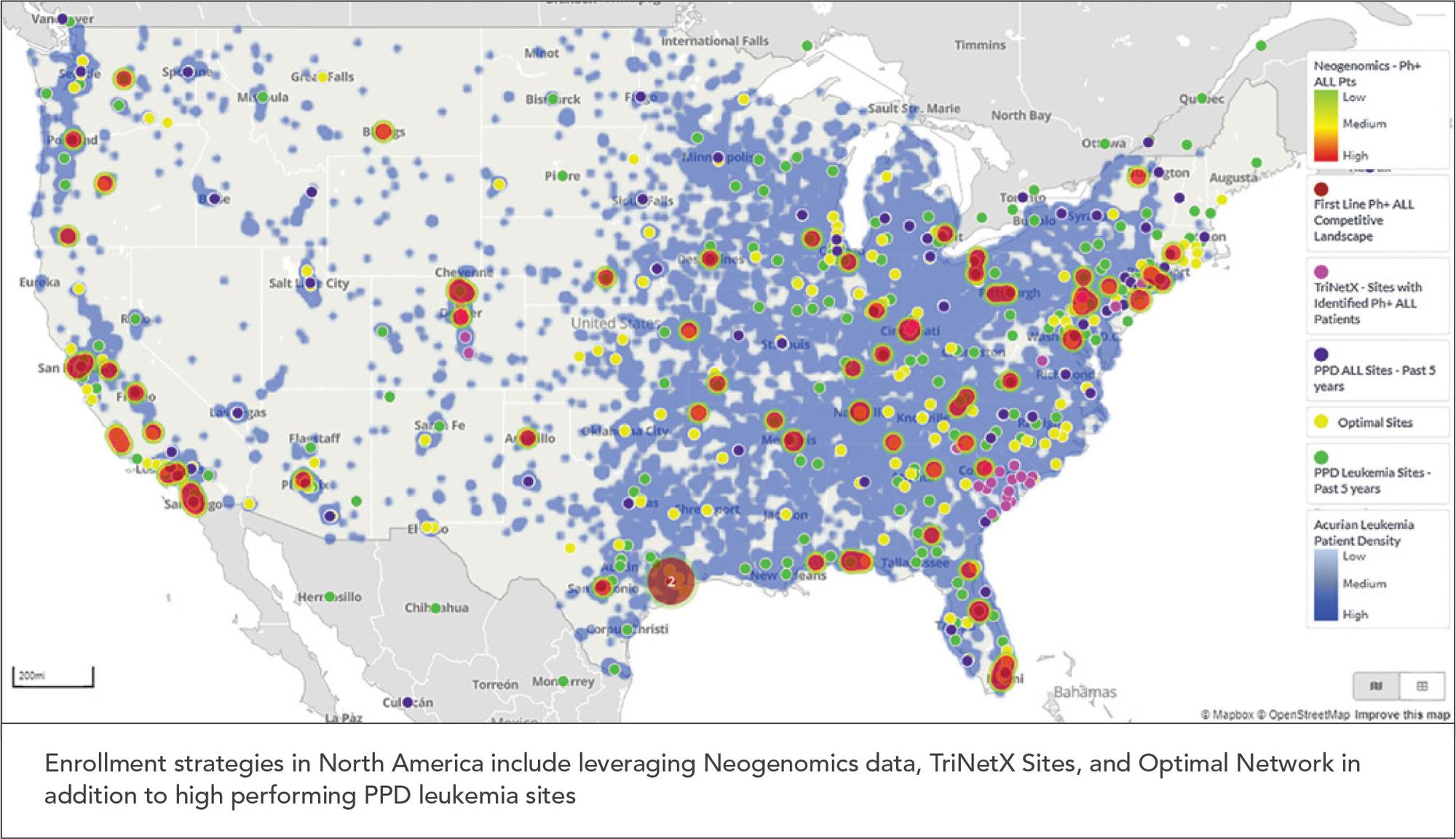
Once data results are generated from these sources, an analysis should be done to marry results of where patients are located with where the most successful sites are located. PPD, for example, can show this visually using our Site and Patient Visualization Tool (SPVT) that provides information regarding the patient populations by number, location, and source (See Figure 2). Identifying sites with the highest population of available patients also is important to reduce patient burden by limiting travel and inconvenience as much as possible.
In the end, the key to site selection is finding the right sites, with the right patients, at the right location to maximize study success and the ability to deliver new therapies to patients in need.
Figure 2. Visual Depiction of Patient Populations and Sites
Protocol De-risking
Protocol de-risking is another strategy built on conducting a set of protocol-focused assessments; however, its goals, timing, and approach differ from protocol optimization (See Table 1). Protocol de-risking is specifically designed to identify and mitigate areas of avoidable risk within the protocol that are likely to lead to protocol amendments.
While a protocol, or protocol synopsis/concept sheet, may undergo a de-risking process at any point, the ideal timing is when the protocol is near-final, just prior to regulatory submission. Performing the analysis at this stage addresses the concern that a large percentage of amendments occur before the first patient is enrolled.6 This timing aims to reduce the probability of receiving competent authority requests for changes, as well as decrease the need for future protocol amendments. As approximately half of amendments are considered avoidable,6,7 identifying and proactively simplifying and modifying the areas of the protocol, that left unchanged most commonly necessitate a future amendment, reduces overall study timelines and costs related to additional submissions and potential pauses in site activations and/or recruitment of patients.
Table 1. Comparison of Protocol Optimization and Protocol De-risking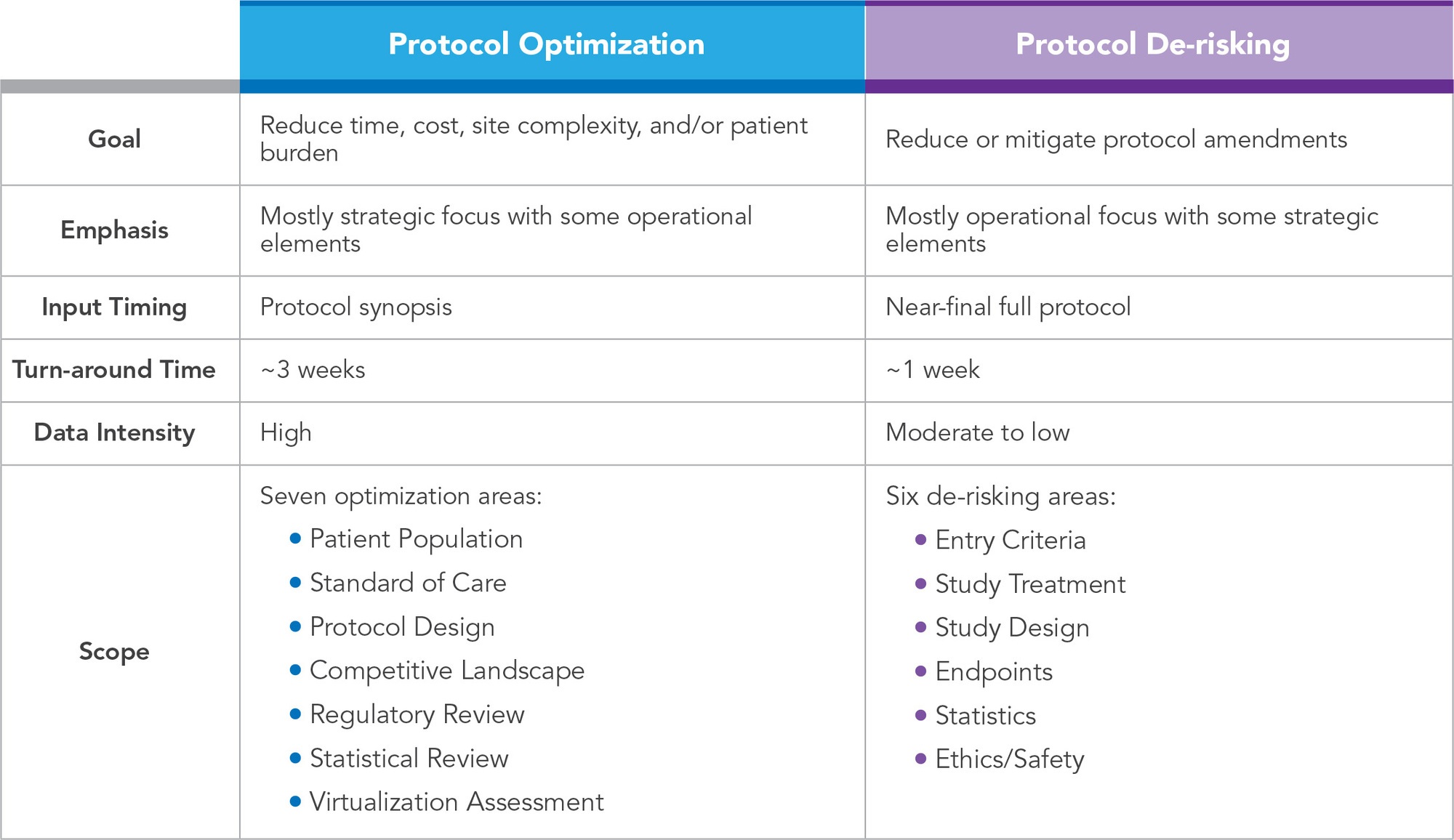
Similar to protocol optimization, protocol de-risking utilizes a team of operational experts with experience in biostatistics, project and clinical management, feasibility, data management, regulatory, pharmacovigilance, medical writing, and product development. The most common causes of avoidable protocol amendments include design flaws, inconsistencies/errors, and recruitment challenges.6-8 The cross-functional team concentrates their analysis and associated recommendation in these areas, as well as customizes the review according to project-specific needs. The following critical data, process, and risks are typically assessed, and associated recommendations provided:
- Clear definitions and alignment between endpoints, objectives, and assessments, and strategy for monitoring critical data
- Eligibility criteria to ensure relevance to study endpoint, clarity, consistency, alignment with applicable guidelines and regional differences, and level of restriction is appropriate for the phase, objectives, and targeted patient population
- Logistical challenges in patient recruitment, retention, study supplies/equipment, investigational product/study treatment, and study procedures
- Study visit assessments to ensure frequency, length, and complexity are appropriate and as minimal as required to meet objectives
- Clarity and comprehensiveness of safety reporting and data collection procedures
- Randomization, stratification, and blinding for feasibility and challenges in execution, as well as completeness needed for statistical analysis plan
- End-to-end consistency review to identify areas of conflict or confusion
Several pilots were conducted to demonstrate the potential benefits of protocol de-risking.
Conclusion
Lowering study complexity and mitigating amendment risk requires a full complement of activities throughout the design process. Effective strategies include starting with early and ongoing patient-informed protocol design, adding protocol optimization and site identification at the synopsis stage, and concluding with thorough protocol de-risking assessments. The impact of these activities is likely to differ from protocol to protocol, but on average, they have demonstrated significant impacts. Lower complexity and fewer amendments mean faster study enrollment and completion, which equates to patients having earlier access to new therapies and companies seeing significant returns on their investment.
References
- Levitan B, Getz K, Eisenstein EL et al. Assessing the Financial Value of Patient Engagement. A Quantitative Approach from CTTI’s Patient Groups and Clinical Trials Project. Ther Innov Regul Sci. 2018 Mar; 52(2): 220–229. doi: 10.1177/2168479017716715.
- US Food and Drug Administration. Patient Engagement in the Design and Conduct of Medical Device Clinical Investigations – Draft Guidance for industry, Food and Drug Administration Staff, and Other Stakeholders. Available at: https://www.fda.gov/media/130917/download. Accessed September 7, 2020.
- Crocker JC, Ricci-Cabello I, Parker A et al. Impact of Patient and Public Involvement on Enrolment and Retention in Clinical Trials: Systematic Review and Meta-Analysis. BMJ. 2018 Nov 28;363: k4738. doi: 10.1136/bmj.k4738.
- Economist Intelligence Unit. The Innovation Imperative. 2019. Available at: https://druginnovation.eiu.com/. Accessed September 7, 2020.
- Levitan B, Getz K, Eisenstein EL, et al. Assessing the Financial Value of Patient Engagement: A Quantitative Approach from CTTI’s Patient Groups and Clinical Trials Project. Ther Innov Regul Sci. 2018 Mar;52(2):220-229. doi: 10.1177/2168479017716715. Epub 2017 Jul 17.
- The Association of Clinical Research Professionals. Are You Wasting Money on Unnecessary Protocol Amendments? April 11, 2017. Available at: https://acrpnet.org/2017/04/11/wasting-money-unnecessary-protocol-amendments/. Accessed September 7, 2020.
- Getz KA, Stergiopoulos S, Short M et al. The Impact of Protocol Amendments on Clinical Trial Performance and Cost. Ther Innov Regul Sci. 2016 Jul;50(4):436-441. doi: 10.1177/2168479016632271.
- Internal Regulatory Affairs Amendment Data from 2017/2018.
For more information, please contact
[email protected], [email protected], [email protected], [email protected], or [email protected].